On June 15, 2011, the research vessel Polarstern (Wikipedia photo right) of the Alfred Wegener Institute for Polar and Marine Research will set off on its 26th arctic expedition.
According to the press release, scientists onboard the vessel plan to take seafloor samples from a marine area in which fishery echosounders recently detected numerous gas flares. They indicate that probably enormous quantities of methane are released from the seafloor at water depths of around 400 metres west of Svalbard.
Scientists have been researching the potential for methane releases in the Arctic for years. One of the dangers with climate change is that hydrates could become destabilized, causing huge amounts of methane to be released, in turn accelerating warming.
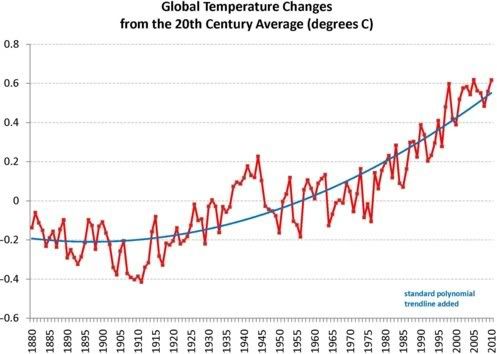
The East Siberian Arctic Shelf is a region about 2,000,000 km2 large that, due to polar amplification of global warming, can now be 10°C or 18°F warmer than it was from 1951 to 1980 (NASA image below).
 Shakhova and Semiletov (2010) conclude that this ESAS region should be considered the most potential in terms of possible climate change caused by abrupt release of methane.
Shakhova and Semiletov (2010) conclude that this ESAS region should be considered the most potential in terms of possible climate change caused by abrupt release of methane.
A press release accompanying a widely-reported study published in Science in 2010 explains that in the shallows of the East Siberian Arctic Shelf, methane simply doesn't have enough time to oxidize, which means more of it escapes into the atmosphere. That, combined with the sheer amount of methane in the region, could add a previously uncalculated variable to climate models.
"The release to the atmosphere of only one percent of the methane assumed to be stored in shallow hydrate deposits might alter the current atmospheric burden of methane up to 3 to 4 times," Shakhova warns.
A 2008 paper by Shakhova et al. considered release of up to 50 Gt of predicted amount of hydrate storage as highly possible for abrupt release at any time. Such a release could multiply the atmospheric methane burden by up to 11 times.
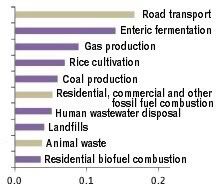
2. Abrupt merthane releases
As permafrost melts, algae and bacteria can flourish, contributing methane through their metabolism. Even more worrying, collapse of methane hydrates can cause abrupt release of huge amounts of methane.
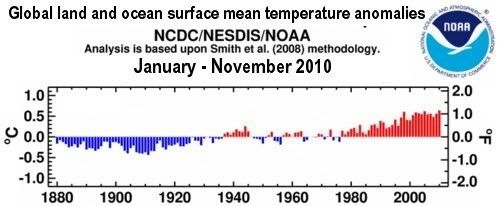
Rising temperatures can cause hydrates to collapse, resulting in abrupt release of huge amounts of methane. Anomolies of up to 12.5°C show up on the image below with average temperatures for November 2010.

For individual days and locations, the anomaly can be even more striking. On January 6, 2011, the minimum temperature in Coral Harbour, located at the northwest corner of Hudson Bay in the province of Nunavut, Canada, was –3.7°C (25.3°F), i.e. 30°C (54°F) above average.
How high can temperatures rise in the Arctic? Below are projections based on above NASA data.

Methane hydrates are held together by high pressure and low temperatures. They can collapse when the temperature rises, or when pressure falls, e.g. when hydrates are disturbed by earthquakes and associated tsunamis, shock-waves and land-slides. Thermal expansion of land and water can put additional stress on areas prone to seismic activity. Furthermore, as ice and glaciers in the mountains melt away, a substantial weight is disappearing, changing pressures that act on the Earth's crust and contribute to seismic activity. This link was confirmed in several scientific studies, such as this one dating back to 2003. Drilling and fracking in these hydrates could make things worse and trigger abrupt releases of huge amounts of methane.
Collapse of a single hydrate can accelerate local warming, in turn causing further hydrates to similarly start adding large amounts of methane to the atmosphere, as further described below.
3. Oxygen depletion
At the moment, methane releases from undersea sediments may still become oxidized in the water. However, a two-part study by Berkeley Lab and Los Alamos National Laboratory shows that, as global temperature increases and oceans warm, methane releases from clathrates would over time cause depletion of oxygen, nutrients, and trace metals needed by methane-eating microbes, resulting in ever more methane escaping into the air unchanged, to further accelerate climate change.
4. Hydroxyl depletion in the air
To make matters even more catastrophic, high methane concentrations will result in an absence of enough hydroxyl in the air for all this methane to be oxidized. A 2009 study by Drew Shindell found that increases in global methane emissions did cause a 26% hydroxyl decrease. Because of this, methane now persists longer in the atmosphere, before getting transformed into the less potent carbon dioxide.
A Centre for Atmospheric Science study suggests that sea ice loss may amplify permafrost warming, with an ice-free Arctic featuring a decrease in hydroxyl of up to 60% and an increase of tropospheric ozone (another greenhouse gas) of up to 60% over the Arctic. This lack of hydroxyl means that methane will persist in the atmosphere for longer at its high global warming potency.
5. Local concentration of methane
Earth has 510,072,000 km2 of surface, or more than 255 times that of the East Siberian Arctic Shelf. Initial concentration of that much methane in the Arctic makes things even worse. While methane can spread out quickly, it will initially be concentrated where it is released. A major methane release in the high Arctic would take 15-40 years to spread to the South Pole. This methane will allow less heat from sunlight in summer to escape into space, while the sun doesn't set. This could therefore cause summer temperatures to rise dramatically in the Arctic, in turn causing further melting and more warming than we're already witnessing now.
6. Methane's high initial Global Warming Potential
Particularly worrying about methane is its high global warming potential, which can be made worse due to the above points, i.e. lack of oxygen in water, resulting in ever less methane oxidation in water, hydroxyl depletion in the air and local concentration of methane. All this may increase methane's global warming potential.
In its first five years, methane is at least 100 times as potent as carbon dioxide as a greenhouse gas (above image below, from a study by Dessus). Abrupt releases of 15 Gt (or Pg) of methane would result in a burden of 20 Gt of methane (since there already is about 5 Gt in the atmosphere). Applying a global warming potential of 100 times carbon dioxide would give this 20 Gt of methane an initial greenhouse effect equivalent to 2000 Pg of carbon dioxide.
By comparison, atmospheric carbon dioxide levels rose from 288 ppmv in 1850 to 369.5 ppmv in 2000, for an increase of 81.5 ppmv, or 174 PgC. What makes things even worse is that this 174 PgC was spread out over the globe, whereas methane from such abrupt releases in the Arctic would - at least initially - be concentrated in a relatively small area.
Extension of methane's lifetime further amplifies its greenhouse effect, especially for releases that are two or three times as large as current releases.
 The graph on the right, based on data by Isaksen et al. (2011), shows how methane's lifetime extends as more methane is released.
The graph on the right, based on data by Isaksen et al. (2011), shows how methane's lifetime extends as more methane is released.
The GWP for methane typically includes indirect effects of tropospheric ozone production and stratospheric water vapor production. The study by Isaksen et al. shows (image below) that a scenario of 7 times current methane levels (image below,medium light colors) would correspond with a radiative forcing of 3.6 W/m-2.
An addition of less than 30 Pg of methane could create such a scenario (i.e. of 7x the methane we're used to having in the atmosphere) and this would extend methane's lifetime to some 18 years, so such a burden will not go away quickly. The situation is even worse when releases take place abruptly over a short period. A single submarine landslide can release 5 Pg of methane, which can double the methane currently in the atmosphere when this occurs in shallow waters, since such a huge release will saturate the water, so most methane will enter the atmosphere unchanged, to trigger further releases.
7. Runaway Global Warming
This kind of warming in the Arctic could result in ever more methane ending up in the atmosphere and remaining there for a longer period without getting oxidized. Initially, all this methane will be concentrated in the arctic, causing huge amplification of the greenhouse effect there in summer, heating up the sea and causing further depletion of oxygen (as algae start to bloom) and further accelerating the permafrost melt and thus causing further carbon to be released from permafrost and clathrates.
Such dramatic local warming is bound to trigger further melting of permafrost locally, resulting in further releases of methane. Massive amounts of methane are stored in the Arctic, much of it concentrated at high density in hydrates. One liter of hydrate can release up to 164 liters of methane. A rise in temperature could cause abrupt releases of huge amounts of methane from hydrates.
8. What can be done about it?
Once runaway global warming starts, it feeds on itself. While dramatic reduction in global greenhouse gas emissions is imperative, that alone will not be able to stop runaway global warming.
Geoengineering methods could reflect some of the sunlight in the Arctic back into space, such as by distributing sulfur dioxide into the stratoshphere by jets, cannons or hoses, or by enhancing cloud albedo as proposed by Stephen Salter and John Latham (see image left).
Even halving the amount of sunlight may not be enough to reduce rapid warming in the region, if that would merely be like cutting methane's GWP in half. Moreover, it can take several years for warming to reach and penetrate hydrate sediments, as described by Nesbit, and once on its way, reducing surface temperature may not be able to reverse such a process quickly enough to avoid massive methane releases. In other words, the window of opportunity for solar reduction methods may already have closed.
Further methods include ways to ignite the methane using short, amplified and focused pulses of UV light from airplanes or satellites. UV light could also be used to produce more hydroxyl, in efforts to oxidize as much methane as possible.
Igniting or breaking down methane may also be possible using model airplanes, equipped with LiPo batteries and with solar thin film mounted both on top of and underneath the wings. Numerous such planes could navigate to the Arctic by autopilot in summer, when there are high concentrations of hydrogen peroxide and when the sun shines 24-hours a day. Flying figure-8 patterns with the wings under an angle could optimize capture of sunlight, keeping the planes in the air, while using surplus energy to power UV lights. Another methods could be to focus UV light on ozone and mix it with volatile hydrocarbons, in an effort to produce hydroxyls. At the end of summer, the planes could return home for a check-up and possible upgrade of the technology, to be launched again early summer the next year.
Such methods are further discussed at this geoengineering group.










 As
As 
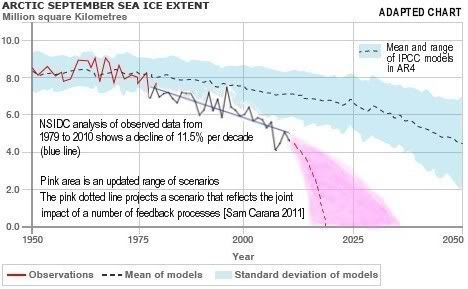
 Arctic sea ice cover in December 2010 was the lowest on record for the month,
Arctic sea ice cover in December 2010 was the lowest on record for the month, 



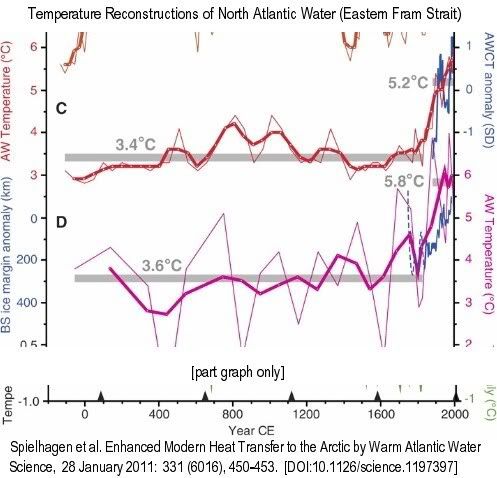 Thin lines are raw data, bold lines are three-point running means…. (C) Summer temperatures at 50-m water depth (red)…. Gray bars mark averages until 1835 CE and 1890 to 2007 CE. Blue line is the normalized Atlantic Water core temperature (AWCT) record … from the Arctic Ocean (1895 to 2002; 6-year averages)…. (D) Summer temperatures (purple) [calculated with a different method]
Thin lines are raw data, bold lines are three-point running means…. (C) Summer temperatures at 50-m water depth (red)…. Gray bars mark averages until 1835 CE and 1890 to 2007 CE. Blue line is the normalized Atlantic Water core temperature (AWCT) record … from the Arctic Ocean (1895 to 2002; 6-year averages)…. (D) Summer temperatures (purple) [calculated with a different method]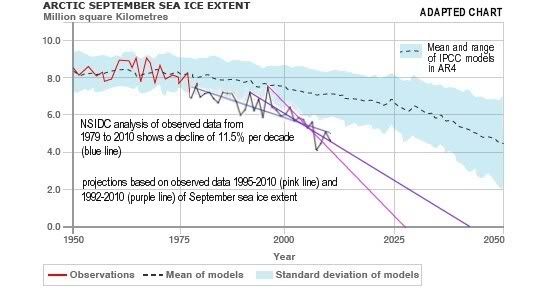



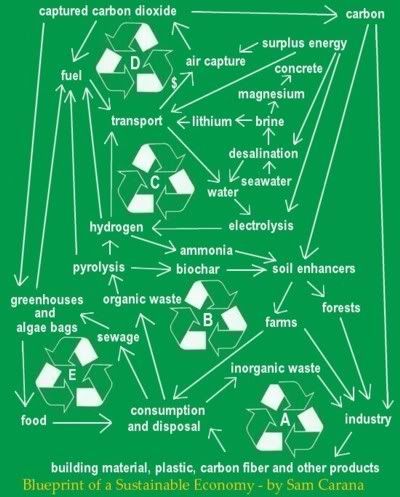
 Additionally, the aviation industry can offset emissions, e.g. by funding air capture of carbon dioxide. The carbon dioxide thus captured could be partly used to produce fuel, which could in turn be used by the aviation industry, as pictured on the left. The carbon dioxide could also be used to assist growth of biofuel, e.g. in greenhouses.
Additionally, the aviation industry can offset emissions, e.g. by funding air capture of carbon dioxide. The carbon dioxide thus captured could be partly used to produce fuel, which could in turn be used by the aviation industry, as pictured on the left. The carbon dioxide could also be used to assist growth of biofuel, e.g. in greenhouses.
 Apart from growing algae in greenhouses, we should also consider growing them in bags. NASA scientists
Apart from growing algae in greenhouses, we should also consider growing them in bags. NASA scientists 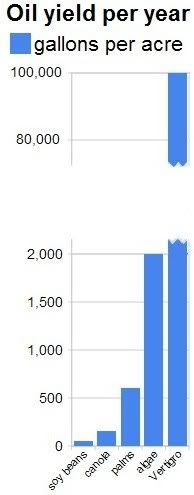 The NASA article conservatively mentions that some types of algae can produce over 2,000 gallons of oil per acre per year. In fact, most of the oil we are now getting out of the ground comes from algae that lived millions of years ago. Algae still are the best source of oil we know.
In the NASA proposal, there's no need for land, water, fertilizers and other nutrients. As
The NASA article conservatively mentions that some types of algae can produce over 2,000 gallons of oil per acre per year. In fact, most of the oil we are now getting out of the ground comes from algae that lived millions of years ago. Algae still are the best source of oil we know.
In the NASA proposal, there's no need for land, water, fertilizers and other nutrients. As 
 A 2007
A 2007