genie back in the bottle. After a half-century of “thinking small” about climate action, we would be forced to think big—big enough to quickly pull back from the danger zone for tipping points and other abrupt climate shifts.
now, we can also judge how close we have already come to painting ourselves into a corner where all escape routes are closed off.
Getting serious about emissions reduction will be the first course of action to come to mind in a climate crisis, as little else has been discussed. But it has become a largely ineffective course of action
with poor prospects, as the following argument shows.
, global average overheating is more than 2°C by 2048. But in the US, we get there by 2028. It is a similar story for other large countries.
Because most of the growth in emissions now comes from the developing countries burning their own fossil fuels to modernize with electricity and personal vehicles, emissions growth is likely out of control, though capable of being countered by removals elsewhere.
But suppose the world somehow succeeds. In the slow growth IPCC scenario, similar to what global emissions reduction might buy us, 2°C arrives by 2079 globally–but in the US, it arrives by 2037.
century, emissions reduction has now become a failed strategy, though still useful as a booster for a more effective intervention.
We must now resort to a form of geoengineering that will not cause more trouble than it cures, one that addresses ocean acidification as well as overheating and its knock-on effects.
would reduce the global overheating, relieve deluge and drought, reverse ocean acidification, reverse the thermal expansion portion of sea level rise, and reduce the chance of more
abrupt climate shifts.
from the air appear inadequate: too little, too late. They do not meet the test of being sufficiently big, quick, and secure. There is, however, an idealized approach to ocean fertilization
that appears to pass this triple test.
It mimics natural up- and down-welling processes using push-pull ocean pumps powered by the wind. One pump pulls sunken nutrients back up to fertilize the ocean surface—but then another pump immediately pushes the new plankton production down to the slow-moving depths before it can revert to CO
growth has been exponential. Excess CO
is that above 280 ppm in the air, the pre-industrial (1750) value and also the old maximum concentration for the last several million years of ice age fluctuations between 200 and 280 ppm.
, allowing a 70 ppm anthropogenic excess, low enough? We hit 350 ppm in 1988, well after the sudden circulation shift
that began in 1978, and the sustained doubling (compared to the 1950-1981 average) of world drought acreage
that suddenly began in 1982.
, so for simplicity I have used 280 ppm as my target: essentially, cleaning up all excess CO
.
temperature has not been observed to suddenly jump, even in the European heat waves of 2003 and 2010. However, other global aspects of climate have shifted suddenly and maintained the change for many years.
The traditional concern, failure of the northern-most loop of the Atlantic meridional overturning circulation (AMOC), has been sidelined by model results
that show no sudden shutdowns (though they do show a 30% weakening by 2100).
While the standard cautions about negative results apply, there is a more important reason to discount this negative result: there have already been decade-long partial shutdowns not seen in the models.
in 1997. Were both the Greenland Sea and the Labrador Sea flushing to fail together
, we could be in for a major rearrangement of winds and moisture delivery as the surface of the Atlantic Ocean cooled above 55
N. From these sudden failures and the aforementioned leaps in drought, one must conclude that big trouble could arrive in the course of only 1-2 years, with no warning.
is not to be confused with climate stability; it still leaves us overheated and in the danger zone for climate jumps. Nor does “stabilized” imply safe.)
While quicker would be better, I will take twenty years as the target for completing the excess CO
cleanup in order to estimate the drawdown rate needed.
The Size of the Cleanup
It is not enough to target the excess CO
2 currently in the air, even though that is indeed the cause of ocean acidification, overheating, and knock-on effects. We must also deal with the CO
2 that will be released from the ocean surface as air concentration falls and the bicarbonate buffers reverse, slowing the drawdown.
Thus, I take as the goal to counter the anthropogenic emissions
4,5 since 1750, currently totaling 350 gigatonnes of carbon. (GtC =10
15g of Carbon=PgC.)
During a twenty year project period, another 250 GtC are likely be emitted, judging from the 3% annual growth in the use of fossil fuels
5 despite some efforts at emissions reduction. Thus we need to take bac
k 600 GtC within 20 yr at an average rate of 30 GtC/yr in order to clean up (for the lesser goal of countering continuing emissions, it would take 10 to 15 GtC/yr).
Chemically scrubbing the CO
2 from the air is expensive and requires new electrical power from clean sources, not likely to arrive quickly enough. On this time scale, we cannot merely scale up what suffices on submarines.
Thus we must find ways of capturing 30 GtC/yr with traditional carbon-cycle
8 biology, where CO
2 is captured by photosynthesis and the carbon incorporated into an organic carbon molecule such as sugar
. Then, to take this captured carbon out of circulation, it must be buried to keep decomposition methane and CO
2 from reaching the atmosphere.
Sequestering CO2
One proposal
26 is to bundle up crop residue (half of the annual harvest is inedible leaves, skins, cornstalks, etc.) and sink the weighted bales to the ocean floor
. They will decompose there but it will take a thousand years before this CO
2 can be carried back up to the ocean surface and vent into the air.
Such a project, even when done on a global scale, will yield only a few percent of 30 GtC/yr. Burying raw sewage
3 is no better.
If crop residue represents half of the yearly agricultural biomass, this also tells you that additional land-based photosynthesis, competing for space and water with human uses, cannot do the job in time.
5 It would need to be far more efficient than traditional plant growth. At best, augmented crops on land would be an order of magnitude short of what we need for either countering or cleanup.
Big, Quick, and Secure
Because of the threat from abrupt climate leaps, the cleanup must be big, quick, and secure.
Doubling all forests might satisfy the first two requirements but it would be quite insecure—currently even rain forests
4 are burning and rotting, releasing additional CO
2.
Strike One. We are already past the point where enhanced land-based photosynthesis can implement an emergency drawdown. They cannot even counter current emissions.
Basically, we must look to the oceans for the new photosynthesis and for the long-term storage of the CO
2 thus captured.
Fertilization per se
Algal blooms are increases in biological productivity when the ocean surface is provided with fertilizer containing missing nutrients
15 such as nitrogen, iron, and phosphorus.
A sustained bloom of algae can be fertilized by pumping up seawater
5,16,19 from the depths, a more continuous version of what winter winds
9 bring up.
Currently about 11 GtC/yr settles out of the wind-mixed surface layer into the slowly-moving depths
13 as plankton die. To settle out another 30 GtC/yr, we would need about four times the current ocean primary productivity. Clearly, boosting ocean productivity worldwide is not, by itself, the quick way to put the CO
2 genie back in the bottle.
Strike Two. Our 41% CO
2 excess is already too large to draw down in 20 yr via primary productivity increases in the ocean per se.
However, our escape route is not yet closed off. There is at least one plausible prospect for an emergency draw down for 600 GtC in 20 yr. It seeks to mimic the natural ocean processes of upwelling and downwelling.
2. Push-pull ocean pipes
Upwelling and Downwelling
Upwelling from the depths is typically caused by winds which push aside surface waters, especially those strong westerly winds in the high southern latitudes that continuously circle Antarctica without bumping into land.
In addition to the heavier biomass (the larger fecal pellets and shells) that can settle into the depths before becoming CO
2, there is downwelling, an express route to the depths using bulk flow. Surface waters are flushed via whirlpools into the depths of the Greenland Sea and the Labrador Sea
23. This downwelling carries along the surface’s living biomass (from bacteria to fish) as well as the dissolved organic carbon (from feces and smaller cell debris).
Note that, in the surface ocean, there is a hundred times more dissolved organic carbon (DOC) than the organic carbon inside living organisms
1. Bacterial respiration produces CO
2 from this DOC that reaches the air within 40 days.
To augment normal downwelling, one could pump surface DOC and plankton into the ocean depths before they become CO
2. Half of the decomposition CO
2 produced in the depths rejoins the atmosphere when the deep water is first upwelled a millennium later. Thanks to ocean mixing in the depths and multiple upwelling sites at different path lengths, it will come back up spread out in time after that initial delay.
There is an even larger spread because the other half (called refractory DOC
17) is somehow protected from becoming CO
2 for a while, even when cycled through the surface layers multiple times.
17 Average radiocarbon dates for DOC in the depths are about 4,000 years, not 40 days.
Thus, if we somehow sink 600 GtC into the ocean depths over 20 years, the return of 600 GtC of decomposition CO
2 to the air is spread out over, say, 6,000 years. That is an average of 0.1 GtC each year, about 1% of current emissions. Such a slow return of excess CO
2 can be countered by slow reforestation or similar measures.
From this analysis, we still have a plausible way out of the climate crisis, even on an emergency basis.
What follows is an idealized example of how we might implement it, using less than one percent of the ocean surface for the next twenty years to do the equivalent of plowing under a cover crop.
5
 |
| Fig. 1. A plankton plantation design using windmill pumps (ref 5), including a fishing lane free of anchor cables. Shading shows the plume of nutrients from a single pump and the plume of organic matter dispersed in the depths. One advantage of windmills is that compressed air can be generated to be pumped into the depths, addressing anoxia problems. Spacing of windmills, however, is subject to the usual limitations of vortices downwind. |
Plowing Under a Cover Crop
In addition to the up-pump of the fertilization-only example, add another wind-driven pump nearby that flushes the surface water back down into even deeper depths before its new biomass becomes CO
2 again.
If we fertilize via pumping up and sink nearby via bulk flow (a push-pull pump)
, we are essentially burying a carbon-fixing crop, much as farmers plow under a nitrogen-fixing cover crop of legumes to fertilize the soil.
Algaculture yields
25 allow a preliminary estimate to be made of the size of our undertaking. Suppose that a midrange 50 g (as dry weight) of algae can be grown each day under a square meter of sunlit surface, and that half is carbon
. Thus it takes about 1 x 10
-4 m
2 to grow 1 gC each year. To produce our 30 x 10
15 gC/yr drawdown rate would require 30 x 10
11 m
2 (0.8% of the ocean surface, about the size of the Caribbean).
But because we pump the surface waters down, not dried algae, we would also be sinking the entire organic carbon soup of the wind-mixed surface layer: the carbon in living cells plus the hundred-fold larger amounts in the surface DOC. Thus the plankton plantations might require only 30 x 10
9 m2 (closer to the size of Lake Michigan).
Apropos location
5, pumping down to 150 m near the edge of the continental shelf would deposit the organic carbon where it could be carried over the cliff and into the slower-moving deep ocean
.
The ocean pipe spacing, and the volume pumped down, will depend on the outflow needed to optimize the organic carbon production
(the chemostat calculation). Only field trials are likely to provide a better estimate for the needed size of sink-on-the-spot plankton plantations, pump numbers, and project costs. The obvious test beds are the North Sea and Gulf of Mexico where thousands of existing drilling platforms could be used to support appended pipes and pumps for field trials
5. Without waiting for floating pumps, we could quickly test for impacts as well as efficient plantation layouts.
I have used windmills here for several reasons: they are familiar mechanisms and they enable a push-pull plantation layout to be readily illustrated. But there are a number of ways to achieve wind-wave-powered pumps, both up and down, such as
atmocean.com’s buoyed pipes and Salter’s elevated ring
23a to capture wave tops and create a hydrostatic pressure head for sinking less dense warm water into the more dense cool waters of the depths. Each implementation will have considerations peculiar to it; what follows are some of the more general advantages and disadvantages in the context.
 |
| Fig 2 A,B: A less expensive pump can be constructed that uses wave power and allows closer packing (ref 3). They would be more effective in the Antarctic Circumpolar Current because of the wave heights. Calvin (2012b), after P. Kithel’s design (atmocean.com). |
 |
| Fig 3. Salter Sink23a uses a meter-high lip on a large floating ring to capture wavetops. This builds up enough hydrostatic pressure to push down warm surface water, kept enclosed by a skirt. It can also (not shown) achieve some upwelling. Warm water exiting in the depths will rise outside the tube, entraining higher-density nutrient-rich cold water. The mix can rise above the thermocline into the surface layer, fertilizing plankton growth. Detail from figure in Intellectual Ventures white paper13a. |
Pro and Con
Here we have an idealized candidate for removing 600 Gt of excess carbon from the air: the sink-on-the-spot plankton plantation that moves decomposition into the thousand-year depths. Push-pull pumping for fertilization and sequestration is relatively low-tech and merely augments natural up- and downwelling processes.
This idealized candidate has some unique advantages compared to current climate strategies: It is big, quick, and secure. It is impervious to drought and holdout governments. It does not compete for land, fresh water, fuel, or electricity. By bringing up cold water from the depths and sinking warm surface water into the thousand-year depths, it cools the ocean surface regionally. And there is a “cognitive carrot,” an immediate payoff every year (fish catch
5, cooling hurricane paths
9a) while growing the climate fix (the 600 GtC emergency draw down).
The idealized example intentionally uses technologies that are too old or simple to be patentable. The industries most likely to benefit would be fishing and the offshore services presently associated with oil and gas platforms.
It is against such advantages that we must judge the potential downsides
5. Concerns voiced thus far include:
- Could we get international agreement fast enough? Continental shelves in the most productive latitudes belong to relatively wealthy countries. Their independent initiatives could quickly establish many plankton plantations just inside the shelf without new treaties.
- Won’t it pollute? Perhaps not as proposed here, using local algae and nutrients in a vertical loop, but the usual considerations would apply should we want to introduce exotic or modified algal species to achieve even higher rates of sinking potential CO2. Toxic blooms are possible during productivity transitions. With floating enclosures rather than plumes, this would change.
- Won’t anoxic “dead zones” form? Shallow continental shelf sites should be avoided because hypoxia will occur from the decomposition of the downwelled carbon soup in a restricted volume. Fish kills occur when anoxia develops more quickly than fish can find their way out of the increasingly hypoxic zone. However, a maintained hypoxic zone will mostly repel fish from entering.
- We don’t know what will happen. The novelty here is minimal, even less than for iron fertilization. Fertilizing and sinking surface waters merely mimics, albeit in new locations or new seasons, those frequently studied natural processes seen on a large scale in winter mixing and in ocean up- and downwelling. There is also prehistorical precedent. The 80 ppm drawdown of atmospheric CO2 in the last four ice ages is thought to have occurred via enhanced surface productivity, triggered by a major reduction in the Antarctic offshore downwelling27 that re-sinks nutrient-rich waters brought to the surface in high latitudes by the circumpolar winds.
- Won’t this just move the ocean acidification problem into the depths? Since the depths are 98% of ocean volume, there is a fifty-fold dilution of the acidity. Were countering out-of-control emissions to continue for a century, depth acidification might be more of a problem.
- Pumping up will just bring up water with higher CO2 than in the surface waters. A depth difference10 of 40 μmol/kg means that upwelling a cubic meter of seawater brings up an unwanted 0.48 g of inorganic carbon. The resulting fertilization will take that CO2 (and more) out of the surface ocean. Also, pumping down the same volume sinks 1 g of potential CO2 as DOC, even without fertilization.
- Aren't you going to run out of phosphate, what currently limits the global ocean productivity to a fraction of its capacity? Up-pump pipes could be sited to bring up bottom waters from the southern oceans that are currently rich in phosphate.
A Second Manhattan Project
Though these objections do not seem insurmountable good reasons usually arise for not implementing most such projects
as initially proposed.
This idealized push-pull ocean pumps proposal is meant to give a concrete example, easy to remember, that defines the response ballpark by being big, quick, secure, powered by clean sources, and inexpensive enough so that a country can implement it on its own continental shelf without endless international conferences. Other drawdown schemes—say, floating enclosures or wave-driven circulating cells — need to pass those same tests.
To do the planning job right is going to take a Second Manhattan Project of various experts to design cleanup candidates and evaluate their side effects. Lend them Los Alamos and let the Pentagon buy them what they need with wartime priorities. To field test their plantation designs, let them instrument the many abandoned oil platforms in the North Sea and the Gulf of Mexico. Then quickly deploy the best designs, using the abilities of the offshore services industry.
Aim to accomplish all this in the four year time frame of the original Manhattan Project. Ten years after that, the cleanup job should be half done, and without all of the economic pain of a quick (and ineffective) shutdown of fossil fuel use. At the beginning of World War II, Franklin D. Roosevelt used the metaphor of a “four alarm fire up the street” that had to be extinguished immediately, whatever the cost. Our need for fast action on climate deterioration requires devoting the resources necessary to radically shorten the developmental cycle for all carbon burial projects. We dare not wait until we are weakened before undertaking emergency climate repairs. Our ability to avoid a human population crash will be compromised if economies become fragile or if international cooperation is lost via conflicts. A serious jolt—say, a major rearrangement of the winds—could cause catastrophic crop failures and food riots within several years, creating global waves of climate refugees with the attendant famine, pestilence, war, and genocide.
Acquiescing in a slower approach to climate is, in effect, playing Russian roulette with the climate gun. The climate crisis needs wartime priorities now.
References
1. Amon RM, Budéus G, Meon B (2003) Dissolved organic carbon distribution and origin in the Nordic Seas: Exchanges with the Arctic Ocean and the North Atlantic. J Geophys Res 14: 1-17.
www.agu.org/journals/jc/jc0307/2002JC001594/2002JC001594.pdf
2. Calvin WH (2008) Global Fever: How to Treat Climate Change. London and Chicago: University of Chicago Press.
faculty.washington.edu/wcalvin/bk14
3. Calvin WH (2008) Estimates for sequestering organic carbon via sinking sewage in oceans.
arXiv 0810.2275v1
4. Calvin WH (2012a) The Great Climate Leap.
ClimateBooks.
5. Calvin WH (2012b) The Great CO2 Cleanup.
ClimateBooks.
6. Dai A, Trenberth KE, Qian T (2004) A global data set of Palmer Drought Severity Index for 1870–2002: Relationship with soil moisture and effects of surface warming. J Hydrometeorology 5:1117-1130.
www.cgd.ucar.edu/cas/adai/papers/Dai_pdsi_paper.pdf
7. Diamond J (2003) Collapse: How Societies Choose to Succeed or Fail. New York: Viking.
8. Falkowski PG, Laws EA, Barber RT, Murray JW (2003). Phytoplankton and their role in primary, new, and export production. In M. J. Fasham (Ed), Ocean Biogeochemistry (ch.4). New York: Springer.
www.ocean.washington.edu/people/faculty/jmurray/chap-04.pdf
9. Feely RA, Sabine CL, Takahashi T, Wanninkhof R (2001) Uptake and storage of carbon dioxide in the ocean: The global CO2 survey. Oceanography 14:18-32.
www.pmel.noaa.gov/pubs/outstand/feel2331/feel2331.shtml
10. Goyet C, Healy R, Ryan J, Kozyr A (2000) Global Distribution of Total Inorganic Carbon and Total Alkalinity below the Deepest Winter Mixed Layer Depths. ORNL Technical report NDP-076 at
www.osti.gov/bridge/product.biblio.jsp?osti_id=760546
11. Greene CH, Baker DJ, Miller DH (2010) A very inconvenient truth. Oceanography 23:214-218.
www.tos.org/oceanography/archive/23-1_greene.pdf
12. Hansen J, et al (2008) Target atmospheric CO2: Where should humanity aim? Open Atmos Sci J 2:217–231. doi:
10.2174/1874282300802010217
13. Houghton RA (2007) Balancing the global carbon budget. Ann Rev Earth Planet Sci 35:313–347.
doi:10.1146/annurev.earth.35.031306.140057
13a. Intellectual Ventures white paper (2009) Drains for hurricanes.
http://intellectualventureslab.com/wp-content/uploads/2009/10/Salter-Sink-white-paper-300dpi1.pdf
14. Joshi M, Hawkins E, Sutton E, Lowe J, Frame D (2011) Projections of when temperature change will exceed 2°C above pre-industrial levels. Nature Climate Change 1:407-412, doi:
10.1038/nclimate1261
15. Lampitt RS, et al (2008) Ocean fertilization: a potential means of geoengineering? Phil Trans Roy Soc A 366:3919–3945.
doi: 10.1098/rsta.2008.0139
16. Lovelock JW, Rapley CG (2007) Ocean pipes could help the Earth to cure itself. Nature 449:403.
doi:10.1038/449403a
17. McNichol AP, Aluwihar LI (2007) The power of radiocarbon in biogeochemical studies of the marine carbon cycle: insights from studies of dissolved and particulate organic carbon (DOC and POC). Chemical Reviews 107:443-466,
doi:10.1002/chin.200724246.
18. Miller AJ, Cayan DR, Barnett TP, Oberhuber JM (1994) The 1976-77 climate shift of the Pacific Ocean. Oceanography 7: 21–26.
meteora.ucsd.edu/~miller/papers/shift.html
19. Oschlies A, Pahlow M, Yool A, Matear RJ (2010), Climate engineering by artificial ocean upwelling: Channelling the sorcerer’s apprentice. Geophys Res Lett 37, L04701, doi:
10.1029/ 2009GL041961
20. Pitman AJ, Stouffer RJ (2006) Abrupt change in climate and climate models. Hydrol. Earth Syst. Sci. Discuss., 3, 1745–1771.
www.hydrol-earth-syst-sci-discuss.net/3/1745/2006/
21. Rahmstorf S (2006) Thermohaline Ocean Circulation. In: Encyclopedia of Quaternary Sciences, edited by S. A. Elias. Elsevier, Amsterdam.
www.pik-potsdam.de/~stefan/Publications/Book_chapters/rahmstorf_eqs_2006.pdf
22. Rahmstorf S, Ganopolski A (1999) Long-term global warming scenarios computed with an efficient coupled climate model. Climatic Change 43: 353–367, doi:
10.1023/A:1005474526406
23. Rhines PB (2006) Sub-Arctic oceans and global climate. Weather 61:109-118. doi:
10.1256/wea.223.05
23a. Salter S (2009) Wave-powered destratification for hurricane suppression, acidity reduction, carbon storage, and enhanced phytoplankton, dimethyl sulfide and fish production. Earth and Environmental Science. DOI:
10.1088/1755-1307/6/5/452012
24. Schlosser P, Bönisch G, Rhein M, Bayer R (1991) Reduction of deepwater formation in the Greenland Sea during the 1980s: Evidence from tracer data. Science 251:1054–1056.
www.sciencemag.org/cgi/reprint/251/4997/1054.pdf
25. Sheehan J, Dunahay T, Benemann J, Roessler P (1996) A Look Back at the U.S. Department of Energy’s Aquatic Species Program—Biodiesel from Algae, NREL/TP–580–24190. US DOE Technical Report.
www1.eere.energy.gov/biomass/pdfs/biodiesel_from_algae.pdf
26. Strand S, Benford G (2009) Ocean sequestration of crop residue carbon: recycling fossil fuel carbon back to deep sediments. Environ Sci & Tech 43:1000-1007.
doi:10.1021/es801555
27. Toggweiler JR, Murnane R, Carson S, Gnanadesikan A, Sarmiento JL (2003) Representation of the carbon cycle in box models and GCMs, 2, Organic pump. Global Biogeochem Cycles 17:1027,
doi:10.1029/2001GB001841
28. Våge K, et al (2009) Surprising return of deep convection to the subpolar North Atlantic Ocean in winter 2007–2008. Nature Geoscience 2:67–72.





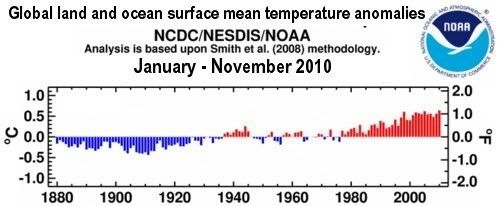
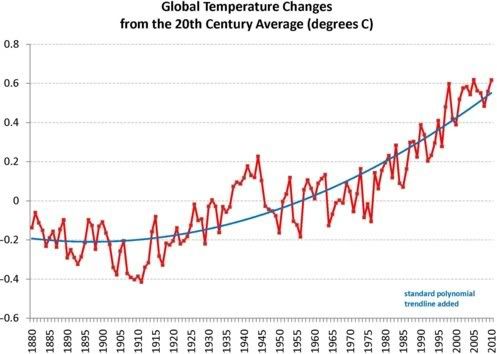
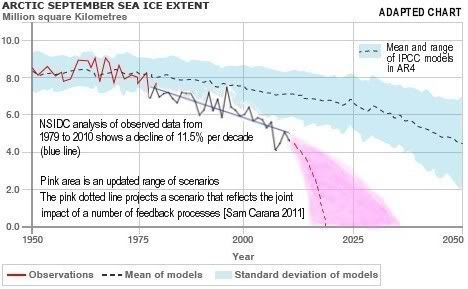
 Arctic sea ice cover in December 2010 was the lowest on record for the month,
Arctic sea ice cover in December 2010 was the lowest on record for the month, 



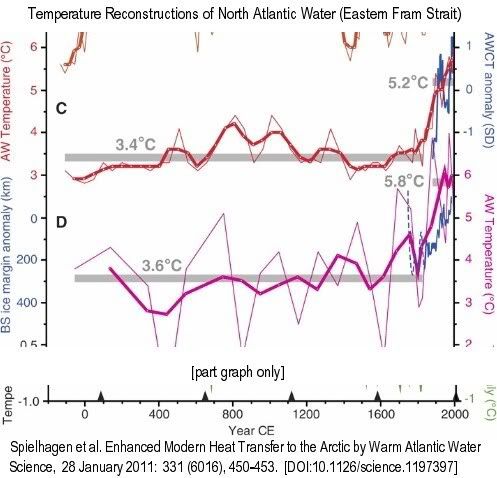 Thin lines are raw data, bold lines are three-point running means…. (C) Summer temperatures at 50-m water depth (red)…. Gray bars mark averages until 1835 CE and 1890 to 2007 CE. Blue line is the normalized Atlantic Water core temperature (AWCT) record … from the Arctic Ocean (1895 to 2002; 6-year averages)…. (D) Summer temperatures (purple) [calculated with a different method]
Thin lines are raw data, bold lines are three-point running means…. (C) Summer temperatures at 50-m water depth (red)…. Gray bars mark averages until 1835 CE and 1890 to 2007 CE. Blue line is the normalized Atlantic Water core temperature (AWCT) record … from the Arctic Ocean (1895 to 2002; 6-year averages)…. (D) Summer temperatures (purple) [calculated with a different method]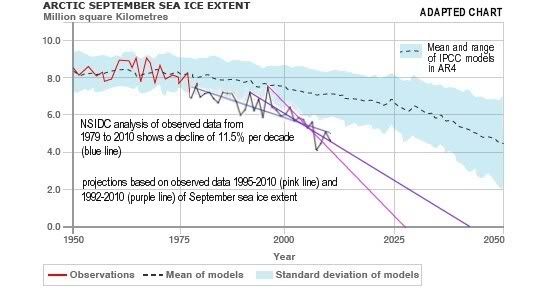


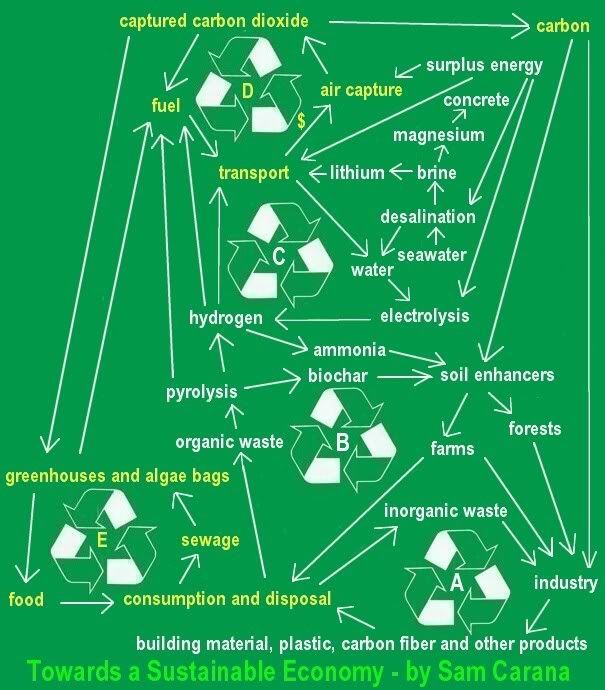
 AIR CAPTURE of CO2 (carbon dioxide) is an essential part of the blueprint to reduce carbon dioxide to acceptable levels. Fees on
AIR CAPTURE of CO2 (carbon dioxide) is an essential part of the blueprint to reduce carbon dioxide to acceptable levels. Fees on  conventional jet fuel seems the most appropriate way to raise funding to help with the development of air capture technology.
conventional jet fuel seems the most appropriate way to raise funding to help with the development of air capture technology.
 Apart from growing algae in greenhouses, we should also consider growing them in bags. NASA scientists
Apart from growing algae in greenhouses, we should also consider growing them in bags. NASA scientists 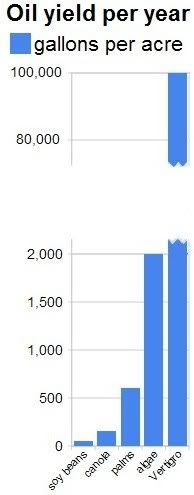 The NASA article conservatively mentions that some types of algae can produce over 2,000 gallons of oil per acre per year. In fact, most of the oil we are now getting out of the ground comes from algae that lived millions of years ago. Algae still are the best source of oil we know.
The NASA article conservatively mentions that some types of algae can produce over 2,000 gallons of oil per acre per year. In fact, most of the oil we are now getting out of the ground comes from algae that lived millions of years ago. Algae still are the best source of oil we know.
 A 2007
A 2007  Professor David Keith (left) of the University of Calgary is working on a tower, 4 feet wide and 20 feet tall, with a fan at the bottom that sucks air in. The tower looks like it's made mainly of plastic, which could be made with carbon produced by such a tower. Inside the tower, limestone or a similar agent is used to bind the CO2 and to split CO2 off by heating it up. The limestone is recycled within the tower, although it does need to be resupplied at some stage. Anyway, the main cost appears to be the electricity to run it.
Professor David Keith (left) of the University of Calgary is working on a tower, 4 feet wide and 20 feet tall, with a fan at the bottom that sucks air in. The tower looks like it's made mainly of plastic, which could be made with carbon produced by such a tower. Inside the tower, limestone or a similar agent is used to bind the CO2 and to split CO2 off by heating it up. The limestone is recycled within the tower, although it does need to be resupplied at some stage. Anyway, the main cost appears to be the electricity to run it.