|
| Aaron Franklin |
By Aaron Franklin
As a compliment to cloud brightening systems, these for use in calm blue sky conditions, or windy blue sky conditions, over Ocean, sea and glacial ice, and land permafrost.
Also may be very important this year for as high tech cloud brightening/making doesn't look like it will be easy to get out in large unit numbers, while there is existing firepump systems that are available in numbers we need now.
Also are essentially no different from snowmaking gear used on ski fields, except for making snow, lower velocity is fine, and no CCN's are required. Just air below 0C, and freshwater.
- High pressure / high volume fire-fighting/water cannon pump gear can be used as is, or modified for higher pressure and kW capacities to increase output volumes at similar nozzle velocities.
An aerated system looks best at this point because:
- By using de Laval nozzles ( convergent-divergent, supersonic and tight stream output ) the aerated water can be accelerated by expansion to high velocity or Supersonic speed as it leaves the divergent exit section of the nozzle.
- Nozzle friction is reduced because air sticks to the surface and creates a gaseous boundary layer.
For Aeration, copper or soft stainless tubes CNC laser perforated, swaged to flare to hexagonal ends, stacked for a honeycomb aeration section (just like a ww2 spitfire radiator except they had the water on the outside of the tubes and no holes) fed with compressed air, in the water feed before the pumps can entrain microbubbles in the water.
- Alternatively supersonic streams can be achieved with unaerated water with convergent nozzles, but more pressure is required.
- The high kinetic energy of the water stream will cause excellent dispersion, and evaporation, via transonic shockwaves as the stream slows, shedding its outer layer as it goes, eventually disintegrating completely either below the altitude where enough kinetic energy, has converted to gravitational potential energy for the stream to go transonic if the stream is below a critical diameter, or not far above that altitude if its above that diameter.
- If its a high velocity Subsonic jet it will still shatter the droplets and evaporate lots of, if not all of them by air turbulence and high differential speed energy conduction/friction evaporation.
- We need to look at freshwater versions as well. This because saltwater rain will be fine over open oceans but it landing on ice and land permafrost will make them melt faster. And saltwater rain on land living ecologies is not at all good either. There's going to be a big use for them to protect the land permafrosts with cloud cover too. Freshwater versions will benefit from using water with diatoms growing in it, as these act as cloud droplet condensation nuclei, just like salt crystals.
Seeding tundra lakes with diatoms will also eat CO2, oxygenate the water enhancing aerobic digestion of dissolved methane and other organic carbon. Removing the diatoms with the water for cloud cannons will also remove excess nutrients from the waters, provide aeration for skyborne digestion of DOC to CO2, and will clean up lakes to make them better for winter snow-making watersources.
- We're going to need to straffe the sky with these things for best cloudmaking effect, so we need to get ready to mount them on naval gun turrets with computer controlled tracking systems and look into parking tanks and APC's with suitable turrets on container ship decks.
Using these tanks and APC's, maybe fixed installations when the wind is blowing, with cloud-cannons on the arctic tundras can help protect the permafrosts.
Calculations and conclusions, for peer review:
These are based on a sonic speed case. Faster will give more range but less volume and slower more volume but less range, for a given pump system.
speed of sound 330m/s
Ep= mgh
Ek= 0.5mv^2
Ek sonic (1 kg water)= 0.5 x 1 x 330^2 = 54450J
54450=mgh=1 x 9.8m/s^2 x h
vertical ballistic altitude h=54450/9.8 = 5.556km
cloud water content = 0.3g/m^3
10m thickness= 3g/m^2
100m thickness= 30g/m^2
4 sqkm= 4,000,000 m^2
Fixed position still air straffing:
A=pi.r^2
r=sqrt(A/pi)
4 sqkm horizontal Cannon range r = sqrt(4/pi)= 1.12km
Moving ship, land tanker, or wind blowing fixed position straffing:
14m/s = 50km/hr (vehicle or wind velocity)
-4 sqkm per hr requires only 4/50= 80m watercannon range.
Water volume and flow rates:
4sqkm at 10m thick= 12000 liters= 12 tons (less than 10min with flow rates of existing fire pumps)
at 100m thick = 120 tons (could be less than an hour per firepump)
1 small Supersonic cloud cannon could produce 24hr x 4sqkm/hr = 96sqkm of 100m thick cloud per day.
Kinetic energy:
120,000 liters per hr / 3600 = 33.3 L/s
12,000 liters per hr / 3600 = 3.3 L/s
Ek Sonic 1kg = 54.45 kJ
kW 100m thick, 4sqkm cloud layer in an hr = 33.3 L/s x 54.45kJ = 1813 kW
- existing pump designs would need to be upgraded for higher power/pressure to produce this much cloud, if supersonic velocities are required, but this is a very small engineering challenge. Ships trawler size and up, and tanks have more than enough kWs for the job. Rapid small amplitude vertical oscillation of the jet release angle should lay down the average 100m thick cloud bank aimed for.
kW 10m thick, 4sqkm cloud layer in an hr = 3.3 L/s x 54.45kJ = 181.3 kW
- this looks good for mobile straffing with existing fire pumps, provided aerated water and deLavel nozzles are used to produce supersonic velocities. The range required for 4 sqkm per hr coverage at only 80m is no problem for the small volume, aerated supersonic water flows possible from existing fire pumps.
Latent heat of evaporation and Ek sonic considerations:
latent heat of evaporation water = 2260 kJ per L
Ek sonic water = 54.45 kJ per L
- If the very small water droplets produced by transonic shockwaves shattering any water breaking from the decaying jet should partially or fully evaporate (this will depend on stream velocity) they will be doing this by absorbing a lot of heat from the air they are landing in. This will cool and supersaturate the air with water vapour, and result in rapid droplet condensation in both saltwater and freshwater versions proposed.
- I am advised that we can expect around 60% humidity levels in arctic conditions. As the evaporative cooling effect will cool the air that the stream droplets land in, and vast quantities of very small cloud nucleation salt crystals will be formed, we can expect a lot more cloud to be formed than the above examples suggest.
- Aeration should result in more and smaller salt crystals, and droplets. In part due to microbubbles enhancing droplet fragmentation. Also due to supersaturation of the water with air, enhanced by evaporation. This causing many disturbances per drop as new bubbles precipitate, and initiate many salt crystals per droplet to precipitate. Turbulence will also initiate precipitation of air and salt crystals in the supersaturated droplet.
- How much extra cloud will depend on how much atmosperic turbulence and mixing is generated by the straffing pattern, and on local temperature and humidity conditions.
- Less mixing will also result in larger cloud droplets.
- Too much mixing will run the risk of forming little cloud at all, as the humidity levels may be too low to form any droplets at all around the salt crystals.
- It's quite likely that 500-600kph will be sufficient velocity. This would produce about 15 litres per second from standard firepump gear. A good estimate seems to be that this would initially produce around 100sqkm of 100m thick cloud bank per day. However from what I am hearing there is likely to be a repeating cycle of droplet evaporation - re nucleation of new droplets - back to droplet evaporation, due to the added water vapour and downwind cooling effects. So total cloud produced may be more than this.
- We should start testing on these ASAP. Others doing testing too, would be a good thing.




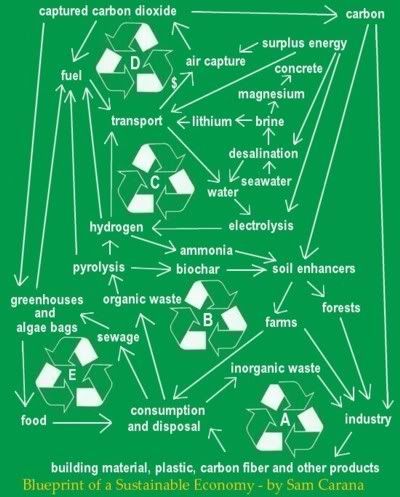
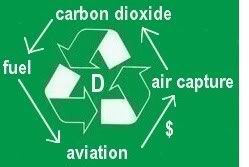 Additionally, the aviation industry can offset emissions, e.g. by funding air capture of carbon dioxide. The carbon dioxide thus captured could be partly used to produce fuel, which could in turn be used by the aviation industry, as pictured on the left. The carbon dioxide could also be used to assist growth of biofuel, e.g. in greenhouses.
Additionally, the aviation industry can offset emissions, e.g. by funding air capture of carbon dioxide. The carbon dioxide thus captured could be partly used to produce fuel, which could in turn be used by the aviation industry, as pictured on the left. The carbon dioxide could also be used to assist growth of biofuel, e.g. in greenhouses.
 Apart from growing algae in greenhouses, we should also consider growing them in bags. NASA scientists
Apart from growing algae in greenhouses, we should also consider growing them in bags. NASA scientists  The NASA article conservatively mentions that some types of algae can produce over 2,000 gallons of oil per acre per year. In fact, most of the oil we are now getting out of the ground comes from algae that lived millions of years ago. Algae still are the best source of oil we know.
In the NASA proposal, there's no need for land, water, fertilizers and other nutrients. As
The NASA article conservatively mentions that some types of algae can produce over 2,000 gallons of oil per acre per year. In fact, most of the oil we are now getting out of the ground comes from algae that lived millions of years ago. Algae still are the best source of oil we know.
In the NASA proposal, there's no need for land, water, fertilizers and other nutrients. As 
 A 2007
A 2007